Bm Beef Jerky for Dogs Warning
Annual Update
Since 2007, FDA has received reports of illnesses in pets associated with the consumption of jerky pet treats.
Complaints
Fanconi-Like Syndrome (FLS)
Testing
Collaboration
Antibiotic Residues & Import Alert Info
Challenges
What You Can Do
Moving Forward
For More Information
COMPLAINTS
As of December 31, 2015, FDA has received approximately 5,200 complaints of illnesses associated with consumption of chicken, duck, or sweet potato jerky treats, many of which involve products imported from China, which produces much of the jerky pet treats on the market. The reports involve more than 6,200 dogs, 26 cats, three people, and include more than 1,140 canine deaths.
We know that the illnesses and deaths reported are most often, but not always, linked to jerky pet treats sourced from China. Pet owners should be aware, however, that manufacturers are not required to list the country of origin for each ingredient used in their products.
The majority of complaints involve chicken jerky (treats, tenders, and strips), but others include duck, sweet potato, and treats where chicken or duck jerky is wrapped around dried fruits, sweet potatoes, yams, or rawhide.
Although it is impossible to conclude definitively in every case whether the events reported were caused by eating jerky pet treats, FDA continues to believe that there is an association between some of the reports and consumption of jerky pet treats. FDA continues to investigate the cause of these illnesses in conjunction with our partners in the Veterinary Laboratory Investigation and Response Network (Vet-LIRN), a network of animal health laboratories affiliated with FDA.
The complaints FDA has received include reported adverse events involving different sizes, ages, and breeds of dogs. About 60 percent of the reports are for gastrointestinal illness (with or without elevated liver enzymes) and about 30 percent relate to kidney or urinary signs. The remaining 10 percent of cases involve a variety of other signs, including convulsions, tremors, hives, and skin irritation.
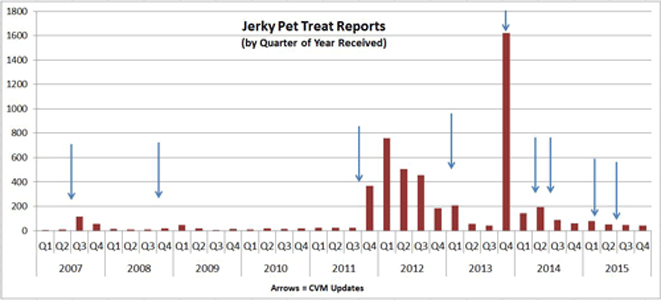
After FDA issued CVM Updates about its Jerky Pet Treats investigation (indicated by the arrows in the graph above), the agency received an increase in reports from the public. The most pronounced increase was in late 2013, when FDA issued our most comprehensive update, which included a "Dear Veterinarian" letter requesting specific clinical data and providing a fact sheet for pet owners. Reported cases appear to be tapering off and have not exceeded 100 per quarter for the past 1.5 years.
Back to the top
FANCONI-LIKE SYNDROME (FLS)
A hallmark of FDA's jerky pet treat investigation has been the unexpected occurrence of cases of acquired Fanconi syndrome (also called Fanconi-like syndrome, FLS), a normally rare kidney disease typically seen primarily in certain breeds as a hereditary condition. Part of the normal function of the kidney is to filter out waste while keeping in the body nutrients such as glucose, bicarbonate, and amino acids. In FLS, a part of the kidney called the proximal tubule doesn't work properly, and these nutrients are lost into the urine instead of being reabsorbed.
Dogs with FLS usually drink and urinate much more than normal. They can also be lethargic and uninterested in eating. Some dogs may have all of these symptoms, while others show only some of them. The symptoms may be mild or severe. These dogs often improve with appropriate veterinary treatment and removal of the pet jerky treats from the diet; however, a positive urine test for FLS can still be detected several weeks later.
Since 2007, FDA received reports of illness for more than 360 dogs that included glycosuria and normoglycemia, characteristic of Fanconi syndrome. Not all of these reports were confirmed with Fanconi testing as being FLS.
Back to the top
TESTING
Testing Jerky Pet Treats
To date, testing for contaminants in jerky pet treats has not revealed a root cause for the reported symptoms in pets. As of Dec 31, 2015, in concert with Vet-LIRN, we have collected approximately 530 jerky treat samples related to more than 290 consumer-related complaints, plus more than 450 retail samples (unopened bags obtained from a store or shipment), and performed a number of tests on these samples.
While we do not subject every sample to the entire battery of testing, due to limited resources and not enough product to run all tests, we target our testing based on the product and the symptoms displayed by the pet. Our product-based testing plans target the main ingredients (e.g. chicken, duck, sweet potatoes), and consider other information on the product label (e.g. additional ingredients such as glycerin or an irradiation symbol).
We continue to revise our testing plan and drop or add new analytes, as new information becomes available.
For example, our past testing included one or more of the following analyses:
- Pathogenic bacteria: Salmonella, B. cereus, S. aureus, C. perfringens; and their enterotoxins: Shigatoxin and S. aureus enterotoxins
- Metals or Elements (such as arsenic, cadmium and lead, etc.)
- Markers of food irradiation (such as 2-alkylcyclobutanones)
- Pesticides
- Antibiotics (including both approved and unapproved sulfanomides, quinolones, and tetracyclines)
- Antivirals (amantadine, rimantadine, oseltamivir, and others)
- Mold and mycotoxins (toxins from mold)
- Rodenticides
- Radioactivity
- Biogenic amines
- Illegal dye agents
- Nephrotoxins (such as aristolochic acid, maleic acid, paraquat, ethylene glycol, diethylene glycol, toxic hydrocarbons, melamine, and related triazines)
- Other chemicals and poisonous compounds
We have removed certain analytes from our testing plan, since these analytes were either found in such extremely small amounts that would not be considered toxic or the analytes were not found at all. Such analytes include biogenic amines, illegal dyes, tanning agents, lactic acid, and hexachlorobutadiene.
As an example, an updated testing plan includes one or more of the following analytes (some of the analytes are still in method development):
- Epichlorohydrin and 3-Monochloropropane-1,2-diol (3-MCPD)
- Glycerin metabolites (such as glyceraldehyde, glycolic acid, diglycolic acid, tartronic acid, and glyoxylic acid)
- Sulfites and Bisulfites (food additives/preservatives)
- Antibiotics (including azitromycin, streptozotocin, and florfenicol)
- Antivirals (including ritanovir)
- Other chemicals and poisonous compounds (such as chaconine, bufotenin, citrinin, 4-Ipomeanol, sulfonamide herbicides, and others)
Testing also included measuring the nutritional composition of jerky pet treats (including glycerol, sorbitol, xylitol, fructose, potassium sorbate, and monosodium glutamate (MSG)) to verify that they contain the ingredients listed on the label and do not contain ingredients that are not listed on the label.
As we mentioned, our product-based testing plans consider the information on the product label, such as the symbol for irradiation. Currently there are no validated methods to determine the dose of radiation that was used to ensure the product was properly irradiated. Until a validated method is available, this aspect of the investigation is pending.
More details on the rationales for our past testing plans, as well as updated testing plans, can be found here:
Vet-LIRN Diagnostic Test Rationales and Results - NEW
Vet-LIRN Diagnostic Test Results for October 2013 - September 2014 (posted February 19, 2015)
Investigation Rationale and Results (posted October 22, 2013)
FDA continues to work with laboratories across the country to investigate causes of reported illnesses in pets that are potentially related to consumption of jerky pet treats. To date, testing for contaminants in jerky pet treats has not revealed a single root cause for the many different reported illnesses.
Dog Testing
Since 2012, in addition to testing jerky pet treats, FDA, in conjunction with Vet-LIRN, has collected and tested urine from dogs reporting various symptoms after eating the treats, to confirm whether dogs that were suspected of having FLS actually had the disease. FDA and Vet-LIRN test the urine from these suspected cases for markers of Fanconi syndrome. As of Dec 31, 2015, FDA had confirmed 214 Fanconi positive dogs out of 263 cases (some cases included multiple animals from the same household). Some of these dogs had not displayed signs of FLS. Some showed gastrointestinal clinical signs, and others were asymptomatic housemates of ill dogs. FDA continues monitoring FLS positive dogs to determine how long markers of FLS stay in the urine after stopping the jerky pet treats. FDA confirmed FLS in 71 percent of dogs (n=145) that were retested approximately 4 months after the initial positive results. FDA's testing and confirmation will help characterize the possible links between exposure to jerky pet treats and developing the rare Fanconi syndrome. This information can help veterinarians recognize, diagnose, and appropriately treat the illness. FDA also uses urine Fanconi testing results to determine which products to test, optimizing our testing resources.
FDA has also had the opportunity to perform necropsies (post-mortem examinations) on dogs suspected of having jerky pet treat-associated illness. We have completed 87 of these as of December 31, 2015. The FDA performed necropsies on as many cases as possible in order to learn more about the cause of death, even when reported symptoms did not appear to be related to eating jerky pet treats. Causes of death other than jerky pet treats were present in more than half (45) of the cases. These causes of death included widespread cancer, Cushing's disease, mushroom toxicity, parvovirus enteritis, bacterial meningitis, abscess, pneumonia, cardiac lesions, loss of blood supply to the tissue, or internal bleeding secondary to trauma. In the remaining 42 dogs, necropsy did not identify specific causes of death and jerky pet treats could not be ruled out or in as contributing to the deaths. Thirty-five dogs had indications of kidney disease, five had gastrointestinal disease, and three had liver disease.
Some of the necropsies (14 of 87) showed kidney tubule changes, an indication of Fanconi syndrome. Dogs diagnosed with FLS usually improve or recover with appropriate veterinary care and removal of the jerky pet treats from the diet; the death rate for FLS is low when treated. Additionally, the necropsies of six of the dogs who tested positive for FLS revealed causes of death unrelated to jerky pet treats.
Although the majority of the dogs reported as testing positive for FLS survived, and their FLS seemed to resolve once the treats were eliminated from the diet, it is important to note that most of these cases also received veterinary care, including hospitalization, intravenous fluids, etc., as part of their treatment.
Back to the top
COLLABORATION
In our efforts to find the cause of the illnesses and deaths associated with jerky pet treats, we've worked with our colleagues in academia, industry, foreign governments, and state labs.
China
As part of the investigation, we have inspected production facilities in China and met with the General Administration of Quality Supervision, Inspection and Quarantine (AQSIQ), the Chinese regulatory agency responsible for pet food, to ensure that they are aware of U.S. requirements for pet food safety and to develop collaboration on sharing information to support FDA's investigation. FDA has also hosted Chinese scientists at our veterinary research facility to further our scientific cooperation.
CDC
Because the Centers for Disease Control and Prevention (CDC) is involved with public health issues that affect both human and animal health, FDA has requested their expertise in collaborating on a study of cases reported to the agency of sick dogs compared with "controls" (dogs that have not been ill). The goal of the study was to compare the foods eaten by the sick dogs (cases) to those eaten by the dogs that did not get sick (controls), in order to determine whether jerky pet treat exposure is associated with illness. Data collected during this investigation will allow federal investigators to better understand what is making pets sick.
Investigators first identified about 100 cases of kidney illnesses in dogs reported to FDA since July 1, 2013, regardless of jerky pet treat exposure. The cases included dogs diagnosed with Fanconi or Fanconi-like illness, or dogs that were 5 years of age or younger and had kidney failure. Cases were selected solely on this case definition and not on what food they consumed. Investigators then identified approximately three control (not ill) dogs within the same geographic region as each sick dog. It took more than 12,000 phone calls to identify fewer than 300 controls. We then interviewed the owners of both the case and control dogs using a detailed questionnaire that included in-depth questions about the types of foods the dogs ate, as well as other factors that could lead to renal disease. A manuscript of study results and analysis is currently being prepared for submission for publication in a peer-reviewed scientific journal.
We are interested in collaborative efforts with veterinary hospitals to perform further study into cases seen in clinical settings to investigate the possibility of a genetic or other basis for gastrointestinal or renal symptoms.
Back to the top
ANTIBIOTIC RESIDUES & IMPORT ALERT INFO
FDA noticed a drop in the number of complaints after several treat products were removed from the market in January 2013 following a study by the New York State Department of Agriculture and Marketing (NYSDAM) that revealed low levels of antibiotic residues in those products. Recalled products included several well-known brands believed to comprise the majority of the jerky pet treat market. Some of these brands returned to the market in February 2014 after formulation changes and FDA has received very few reports (primarily with gastrointestinal symptoms) associated with the new products. After completing a Health Hazard Evaluation, FDA believes it unlikely that the reports of illness it has received were caused by the presence of antibiotic residues in jerky pet treat products. FDA scientists closely reviewed the NYSDAM findings and noted that when measurable levels of antibiotic drugs were found in the treats, they were consistently at very low levels – almost all were less than 0.0001 percent (< 1 part per million, or less than one inch in 16 miles). Our investigation continues to evaluate all potential causes for illness from the jerky pet treats, including antibiotics.
Following testing performed by the NYSDAM that detected low levels of antibiotics in jerky pet treats, FDA undertook a project to adapt the NYSDAM method to the equipment in its own field laboratories for regulatory and enforcement purposes. This adaptation is now complete and the method is in use for testing both imported and domestic treats.
During FY 2014 (October 2013 - September 2014), FDA's Vet-LIRN tested 71 investigative jerky pet treat samples and identified 27 that tested positive for amantadine. FDA did not request a recall of these treats because they had been sold a year or more ago. Based on these findings, FDA validated the methodology and published the methods as a Laboratory Information Bulletin. FDA has added testing for antiviral residues to its sampling assignment and implemented an Import Alert directing its field investigators to detain shipments of the particular products that tested positive. These products cannot enter the country unless the manufacturer or shipper can provide third-party documentation that the products do not contain illegal antiviral and/or antibiotic residues.
FDA does not believe that amantadine contributed to the illnesses because the known side effects or adverse events associated with amantadine do not seem to correlate with the symptoms seen in the jerky pet treat-related cases. However, it should not be present in jerky pet treats. The agency has notified Chinese authorities that FDA considers the presence of amantadine in these products to be an adulterant. FDA has also notified the U.S. companies that market jerky pet treats that tested positive for amantadine.
FDA's Office of Regulatory Science expanded the testing methodology in 2015 to include duck jerky. The expanded testing led to detection of antibiotic and antiviral residues in an imported duck jerky product. The Import Alert was revised in October 2015 to include all poultry jerky-type pet treats.
Back to the top
CHALLENGES
This investigation continues to be a challenging one for FDA. Complicating the investigation are some fundamental differences between investigations into illnesses in people versus those in pets.
In human illness outbreaks caused by foodborne bacteria or contaminants, FDA works in concert with the Centers for Disease Control and Prevention (CDC) and state boards of health, which collect and track cases of foodborne illness. Unfortunately, there is no equivalent for pets, which means that it is difficult to accurately evaluate the scope of an outbreak. For instance, FDA is unaware of any statistics on the background rate of Fanconi or FLS in non-Basenji breed dogs. Without such a baseline, it's hard to appreciate how unusual the findings of Fanconi syndrome might be.
In the Basenji, and some other breeds such as the Norwegian Elkhound, Fanconi Syndrome is usually a genetic condition that can be passed down from parents to offspring. Very little is known about the possible causes for non-genetically related (acquired) Fanconi Syndrome cases in dogs, but certain toxins, medications and infections have been linked to its development in dogs and people.
Another complicating factor in the investigation is the lack of adequate post-mortem information in most cases. When a person dies unexpectedly, it is not unusual for a medical examiner to perform an autopsy to try to determine the cause of death. When a pet dies, it is much less likely that qualified veterinary pathologists will have the opportunity to examine the body. By the time FDA receives reports of deaths in pets, the body often has already been cremated or buried, eliminating the chance for scientists to gather more information about potential causes for the pet's illness. We have had the opportunity to perform necropsies on some dogs in response to our Dear Veterinarian letter, and we are extremely grateful to the owners for their generosity in consenting to these procedures.
Finally, FDA has limited access to market data about food items for pets. FDA regulations do not require product registration for foods, whether they are intended for people or animals. Therefore, the Agency has challenges when determining the scope of the jerky pet treat market and the different products available to consumers.
Back to the top
WHAT YOU CAN DO
Pet Owners
Jerky pet treats are not required as part of a complete and balanced diet for your pet. If you choose to feed jerky pet treats, watch your pet closely. Signs that have been reported in association with JPT may occur within hours to days of feeding the jerky treat products are decreased appetite, decreased activity, vomiting, diarrhea (sometimes with blood or mucus), increased water consumption and/or increased urination. Severe cases may be diagnosed with pancreatitis, gastrointestinal bleeding, and/or kidney failure or the resemblance of Fanconi syndrome.
If you believe your pet has become ill from consuming a jerky pet treat, please provide us with valuable information by reporting it electronically through our Safety Reporting Portal or to your local FDA Consumer Complaint Coordinator. In addition to your contact information, your pet's symptoms, and medical records, the one piece of information we most often lack is the lot number of the jerky treat product. If we have the lot numbers, we can identify whether particular lots triggered more complaints, trace products back to specific manufacturing facilities, and identify lots for testing. While we still want to hear from you even without the lot number, this information can help our investigation immensely.
FDA will determine whether there is a need to conduct a follow-up phone call or obtain a sample of the jerky pet treat product in question. While FDA does not necessarily respond to every individual complaint submitted, each report becomes part of the body of knowledge that helps to inform FDA on the situation or incident.
If you find it convenient to transfer pet food and treats to a secondary container to protect them from rodents, insects, or spoilage, FDA recommends that you consider saving the original packaging. This will help ensure that you will still have access to the lot code if your pet becomes ill from consuming the product.
While working with your veterinarian to review your pet's records, FDA and Vet-LIRN scientists might request specific testing to try to narrow down the cause of your pet's illness. The costs of tests requested by the scientists will be covered by Vet-LIRN and FDA, but pet owners will not be reimbursed for any additional testing expenses they may incur.
Although it is always a difficult topic to consider, in the event of a pet death that appears to be related to the consumption of jerky pet treats, post-mortem testing of animal tissues, such as a necropsy (in human medicine, doctors call this procedure an autopsy) may also be helpful. While we want to do everything we can to prevent pets from becoming ill in the first place, having the chance to examine tissues may fill gaps in information that can help us pinpoint a cause for the reports of injury and death.
Veterinarians
FDA has been investigating the root cause of these adverse events since 2007, and has issued several consumer updates advising pet owners about complaints associated with jerky treats, also noting that such treats are not essential for nutrition. Despite these warnings, we have continued to receive reports of illnesses in both dogs and cats.
In October 2013, in an effort to expand the amount of information included in case reports, we reached out to all licensed veterinarians through a "Dear Veterinarian" letter. The letter, among other things, let veterinarians know in advance what specific types of information would be most useful to FDA when they report cases of suspected jerky pet treat-related illness to the agency. This information includes:
- How long the owner has been feeding the treat
- What else the pet has been eating (all treats, human food, and pet food), including how much is given daily of all items
- Bloodwork values, especially for liver and kidney
- Urinalysis results
FDA also requests in the letter that veterinarians obtain a urine sample (10 ml if possible) from dogs or cats that may have illness associated with jerky pet treats and freeze it for testing for FLS by Vet-LIRN. This testing will allow FDA to get a better idea of how many of the suspected cases involve FLS, whether or not the pets display symptoms of kidney or urinary disease.
Back to the top
MOVING FORWARD
Since 2007, FDA's Center for Veterinary Medicine (CVM) has dedicated increasing resources to pet food, and CVM continues to work diligently to find the cause for illnesses and deaths linked to jerky pet treats.
As veterinarians, animal scientists, and animal lovers ourselves, we strive to make sure that the products FDA's Center for Veterinary Medicine regulates are safe, effective, and properly manufactured. We understand the love and devotion pets provide, and we are determined to find the answer to this mystery.
Back to the top
FOR MORE INFORMATION
Complaints
Complaints come into the FDA through two pathways: through regional consumer complaint coordinators in each of FDA's district offices and through the Safety Reporting Portal.
- Reports from the Safety Reporting Portal (SRP)
- 2010 Reports
- 2011 Reports
- 2012 Reports (through January 2013)
- February - September 2013 Reports
- September 2013 - May 2014 Reports
- May - September 2014 Reports
- Reports from FDA's Regional Consumer Complaint Coordinators (RCCC)
[Note: The RCCC spreadsheet lists a "country of origin" (C.O.O.) column. A few entries list the United States as the C.O.O. This is not correct. The distributor is located in the U.S. but the manufacturer is located in China. In addition, one entry lists, "Afghanistan," as the C.O.O. It should indicate, "China," in the C.O.O. column. Freedom of Information laws require records to be released "as is" regardless of any perceived errors (either manual or human).]
Back to the top
News
- FDA Provides Update on Jerky Pet Treat Investigation - May 16, 2016
- FDA Voice: Help Us Find Out Why Jerky Treats Are Making Pets Sick
- FDA Voice: Is It Something My Pet Ate?
Product Safety Information
- FDA Jerky Pet Treats Investigation (video)
- Why Are Jerky Treats Making Pets Sick?
- Jerky Pet Treats - Letter to Veterinarians
- FDA Report Regarding Jerky Pet Treats and Illnesses
- Vet-LIRN and Jerky Pet Treats Investigation
Firm Inspection Information
- Establishment Inspection Report - Acidchem
- Establishment Inspection Report - Yantai Aska Food
- Yantai Aska Foods 483
- Establishment Inspection Report - Gambol Pet Products Co.
- Establishment Inspection Report - Jinan Uniwell Pet Food Co., Ltd.
- Establishment Inspection Report - Shandong Honva Food Co. Ltd.
- Establishment Inspection Report - Shandong Petswell Food Co. Ltd.
Additional Resources
- How to Report a Complaint about Jerky Pet Treats
- Safety Reporting Portal
meisnerbobbled1985.blogspot.com
Source: https://www.fda.gov/animal-veterinary/outbreaks-and-advisories/fda-investigates-animal-illnesses-linked-jerky-pet-treats
Belum ada Komentar untuk "Bm Beef Jerky for Dogs Warning"
Posting Komentar